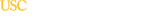The safety of participants and investigators is of paramount importance to us at the CIA. For this reason, the CIA uses multiple levels of access controls to ensure that only authorized, qualified personnel operate equipment, especially the MR systems, and that only those individuals and equipment who have undergone proper screening are permitted within restricted areas.
All scanning sessions require a minimum of two trained personnel with their credentials approved by the CIA.Investigators wishing to scan at the CIA must complete safety certification, which is described here and must learn and follow the procedures outlined below.
I. Purpose
II. MRI Safety Committee
III. Building safety guidelines
IV. Screening procedures
V. Safety certification procedures
VI. Educational training
VII. Media
IX. Standard Operating Procedures
X. Modes of Operation for MR Systems
XI. Special populations
XII. Safety Equipment MR Safe and MR Conditional
XIII. Safety equipment NOT MR Safe or MR Conditional
XIV. Incidental findings
XV. Quality Control Procedures
XVI. Infection control
XVII. Quench procedures
XVIII. Electrical shutdown procedures
XIX. Emergency Procedures
XX. Emergency Contacts
Contraindications for MRI
The purpose of the MRI Safety Policies and Procedures is to maintain safe practice during research and clinical procedures, in the magnetic resonance (MR) imaging areas affiliated with the Center for Image Acquisition (CIA). It has been reported by others that MR-related injuries, fatalities, and equipment damage were the apparent result of failure to follow established safety guidelines. For the purpose of developing and maintaining safe MRI practices, recommendations from the American College of Radiology Guidance Document on MR Safe Practices: 2013 and 2019, http://www.mrisafety.com, information obtained from the peer-reviewed literature, and other sources is used. Because MRI technology continues to progress, this is a living document that is reviewed and updated on an annual basis.
The MRI Safety Committee is responsible for ensuring that the MRI safety guidelines are established and maintained on a current basis and as appropriate for the various MR systems operated.
-
Committee membership
The MRI Safety Committee consists of four members, plus a chair, who is the Director of the Center for Image Acquisition (CIA). -
Committee roles and responsibilities
-
Policy review
The policies and procedures is reviewed and updated annually. Introduction of any substantial changes in MRI system hardware or software that will significantly change the safety parameters in the MR imaging environment (e.g. adding faster/stronger gradient capabilities, higher RF duty cycle sequences) is reviewed prior to implementation. In this review process, national and international standards and recommendations is taken into consideration prior to establishing our own local guidelines, policies, and procedures. -
Incident reports
All adverse MRI safety incidents (or near incidents) that occur in the MR site will be reported to the Chair of the MRI Safety Committee in written form within a 24 hour period. All incidents and adverse effects will be discussed at a quarterly meeting of the MRI Safety Committee. A serious adverse event will result in an immediate session of the MRI Safety Committee.
-
Policy review
-
Access restrictions
For everyone's protection, the Center for Image Acquisition has restricted access to sensitive areas to those personnel with proper safety certification. As outlined below, the scanner control rooms and scanners themselves are insulated by multiple levels of physical access control.
-
Zone I
This area is freely accessible to the general public. This area is the reception and waiting areas. -
Zone II
This area is the interface between the publicly accessible, uncontrolled Zone I and the strictly controlled Zones III and IV. Participants are greeted in Zone II and are not free to move about Zone II at will, but are required to be under the supervision by MR Personnel with a safety certification of Level 1 at all times. -
Zone III
This area is the region in which free access by unscreened non-MR personnel or ferromagnetic objects or equipment can result in serious injury or death as a result of interactions between the individuals or equipment and the MR scanner environment. All access to Zone III is to be strictly restricted with access to regions within it controlled by and entirely under the direct supervision of (escorted by) MR personnel with a safety certification of Level 2. Zone III requires key card access. -
Zone IV
This area is synonymous with the MR scanner room itself, i.e. the physical confines of the room within which the scanner is located. No individual is allowed in the scan room without being supervised by MR personnel with a safety certification of Level 2. The scan room door is always locked when unattended. Only MR Safe or MR Conditional equipment approved by the MR Safety Committee may be brought into Zone IV. The MR technologists must be able to directly observe and control via line of sight the entrances or access III. -
After hours access
All zones require key access on holidays or after normal operating hours, which are defined as Monday-Friday, 8am-5pm, excluding University holidays. Key access is granted upon successful completion of MR Safety Certification Level 2.
For information about obtaining safety certification for Level 1 and Level 2, see: V. Safety certification procedures on this page.
-
Zone I
-
Device and object screening
Only MR safe or MR conditional equipment and devices may enter the scanner room (Zone IV).
- Guidelines). All individuals, including volunteer research participants, patients, visitors, technologists, researchers, ancillary support staff, custodial workers, and maintenance and service providers, must be oriented and verbally pre-screened for MRI safety prior to admittance to Zone III or higher.
-
Screening procedure
Persons wishing to enter Zones III or IV must complete an MRI screening form (Metal Screening Form), reviewed by an MR Operator with Level 2 training. Individuals with any contraindication for MRI must be cleared by the CIA Director for access in Zones III or IV. -
Procedure for removal of metallic objects
The procedure for removal of metallic objects takes place in the Screening location in Zone II. Persons undergoing this procedure must remove all readily removable metallic personal belongings and devices on or in them (e.g., watches, jewelry, body piercing if removable, contraceptive diaphragms), metallic drug delivery patches (i.e., with the knowledge and approval by the prescribing physician), and clothing items which may contain metallic fasteners, hooks, zippers, loose metallic components, metallic threads, etc. Lockers will be provided by the CIA to hold any valuables. No person may enter Zones III or IV without proper screening for metal objects on their person. -
Visitor
A visitor is defined as any individual without any or with incomplete training related to MR safety, human subject participation or experimental animal research participation. This includes students, members of the media, site employees, and parents, family members, or caretakers of the MR participant. Visitors are restricted to Zones I or II unless the de-metaling and screening procedures have been successfully completed. Visitors may not enter Zone IV without prior written permission from the MRI Safety Committee. Exceptions include: caretakers of vulnerable participants or medical personnel. -
Participant
A participant is anyone who is undergoing an MRI procedure. A participant may be either a research subject, defined as any individual who provides informed written consent to participate in approved research protocols, or a patient, defined as any individual who is examined by MRI for clinical diagnostic purposes. All participants undergo de-metaling and screening procedures, and are always to be under the direct supervision of an MR Operator with Level 2 MR safety training in Zones II and III and in the line of sight of a an MR Operator with Level II in Zone IV. -
Accompanying personnel
Although any age participant might request that others accompany them for their MR examination, this is far more common in the pediatric population. Those accompanying or remaining with the participant should be screened using the same criteria as anyone else entering Zone IV. In general, it is prudent to limit accompanying adults to a single individual. Acoustic noise protection and MR Safe/MR Conditional seating are provided for accompanying family members and personnel within the MR scan room. -
MR personnel
All MRI Personnel are to undergo an MRI screening process as part of their employment interview process to ensure their own safety in the MRI environment. A signed, hospital-approved screening form will be kept on file for all MRI Personnel. For their own protection and for the protection of the ancillary staff, all MRI Personnel must immediately report to their supervisor any trauma, procedure, or surgery that they experience, in which a ferromagnetic metallic object or device may have been introduced to their person. This will permit appropriate safety screening to be conducted. -
Clothing restrictions
There have been reports of thermal injuries or burns associated with clothing containing electrically conductive materials, such as metallic threads, electrically conductive designs, and silver-impregnated clothing. The CIA will provide gowns or scrubs as an option to participants; however, it is up to the individual completing the screening procedure to decide whether their use is necessary. Participants should be advised prior to a study to wear appropriate clothing, such as:- Minimize zippers, buckles and snaps on pants.
- Remove belts and other metallic objects.
- Remove shirts, blouses or sweatshirts that have metal zippers, buckles or snaps. Plain shirts, blouses or t-shirts are preferred.
- Under-wire bras or bras with metal clips should be removed. Sports bras are fine.
- Remove steel-toed boots or shoes and shoes with metal or magnets.
- Remove hairpins, barrettes or other metal hair items.
- Note that some "sports clothing" may contain metallic material and, therefore, may cause burns under certain MRI conditions.
All scans must be conducted by a minimum of 2 individuals with MR safety certification, and at least one must have Level 2 MR safety training certification. The CIA MRI Technologist may count as this Level 2 certified person during normal scanning hours, as explained below.
- MRI Technologist
An MRI technologist will be provided during normal business hours, Monday-Friday, 8 am to 5 pm. All MRI technologists will be in compliance with the technologist qualifications listed in the MRI Accreditation Program Requirements. This means that during business hours, at least one additional person must be present who has at least Level 1 certification for a scan to occur.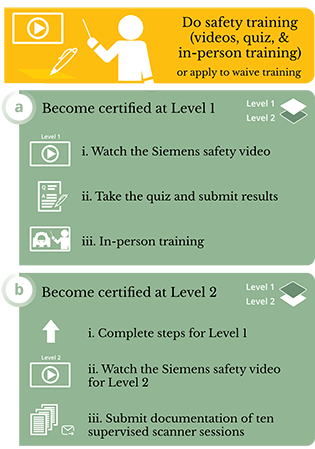
-
MR Operator - Level 1
Level 1 MR personnel: Those who have passed minimal safety educational efforts to ensure their own safety as they work within Zone III will be referred to henceforth as MR Operator - Level 1 MR personnel. Procedure for becoming Level 1 certified:- View this video and pass the quiz with 100%: Basic MRI Safety
- Review the CIA MRI Safety Policy document & take the quiz, provided by and pass with 100%.
- Schedule and successfully complete an orientation to our facilities with MRI technologist.
-
MR Operator - Level 2
In addition to the requirements for Level 1, the following are the procedures to achieve Level 2 certification:- View this video and pass the quiz with 100%: Advanced MRI Safety
- Fulfill the experience requirement, by conducting at least 10 full scanner sessions (minimum 1 hour each) under the supervision of a Level 2 user or MRI technologist, with each session (date, time, scan number, scanning protocols used) signed off on by the supervising party. Level 2 user training requirements must be met for each scanner used (e.g., separate training required for 3T and 7T scanners).
-
Renewal
MR Safety certification must be renewed annually to continue use of the scanner.
Students will require all aspects of Level 1 training, except the orientation. Students must be accompanied by instructor or another person with Level 2 safety certification in any restricted zones, as explained above.
VII. Media
Visiting members of the media must be approved by Dr. Toga, who will assign an escort. Visitors will remain with their escorts at all times while in restricted areas.
-
Measures to minimize risk/discomforts
-
Acoustic noise protection
Anyone in the scanner room (Zone IV) while the scanner is in operation must be provided with and must use hearing protection in the form of earplugs and/or headphones to avoid hearing injury from the acoustic noise generated by the scanner. According to the Prisma documentation, if you are using the Siemens headphones you MUST also provide the subject with earplugs for additional hearing protection. The FDA requires 30 dB or greater for hearing protection and the Siemens headphones alone only provide 13 dB. -
Emergency squeeze bulb
The scanners are equipped with a squeeze bulb that allows the subject to set off an audible alarm to attract the operator's attention. The squeeze bulb should be made available to subjects unless some alternative method of constant monitoring (e.g., another person in the scanner room) is in effect. Use of the squeeze bulb or some comparable form of continuous subject monitoring is mandatory. -
Communication with investigators
The Siemens 3T Prisma comes standard with an intercom system that allows for communication between the participant the MR Operator. -
Laser light
On the 3.0 Tesla Prisma scanner, a laser is available for landmarking the participant's position in the scanner. Subjects should be instructed to keep their eyes closed while the laser light is turned on to avoid eye injury. -
Door security
Scanner room doors are to be closed and pressurized while the scanner is in operation. This not only prevents unauthorized entry to the scanner room, but also reduces the possibility of RF artifacts compromising the quality of the scan. -
Accurate entry of subject height, weight, age, sex
The scanners require that the subject's height, weight, age and sex be entered before scanning. Accurate information must be provided to ensure that FDA limits for energy deposition are not exceeded. Weights should be correct to within five pounds. Incorrect information should never be entered in an effort to get the scanner to conduct a study that it otherwise would not perform because FDA limits would be exceeded.
The FDA guideline is that there is no significant risk if the specific absorption rate (SAR) is:
- Less than 4 W/kg whole body average for 15 minutes,
- Less than 3 W/kg averaged over the head for 10 minutes,
- Less than 8 W/kg in any gram of tissue in the head or torso for 5 minutes, or
- Less than 12 W/kg in any gram of tissue in the extremities for 5 minutes.
-
Temperature control
In regulating energy deposition in the subject's body in accordance with FDA guidelines, the scanners assume that the ambient temperature in the room does not exceed 72° and that the relative humidity does not exceed 60%. Consequently, the thermostat should never be set for a room temperature higher than 72°. Blankets are available for subject comfort if needed. Please note that only cotton, linen or paper should be used for bed covering or blankets since radiofrequency energy may cause heating of synthetic sheets or blankets. -
Minimize subject anxiety or claustrophobia
For certain participants that undergo magnetic resonance (MR) examinations, the experience may be associated with emotional distress. Referring physicians, radiologists, and MRI technologists can best manage affected participants by understanding the etiology of the problem and knowing the appropriate maneuver or intervention to implement in order to counter-act the condition.
The experience of "psychological distress" in the MR environment includes all subjectively unpleasant experiences attributable to the procedure. Distress for the participant can range from mild anxiety that can be handled with simple reassurance, to a more serious panic attack that may require psychiatric intervention or medication. Severe psychological reactions to MR examinations are characterized by the rapid onset of at least four of the following: nausea, paresthesias, palpitations, chest pain, faintness, dyspnea, choking sensation, sweating, trembling, vertigo, depersonalization, and fear of losing control or dying.
The following is a list of various procedures that can be used to minimize distress or anxiety in participants undergoing MR procedures. Some measures should be employed for all examinations, while others may be required only if the participant experiences distress due to the factors described above.- Prepare and educate the participant concerning specific aspects of the MR procedure (e.g., MR system dimensions, gradient noise, intercom system, constant presence of the MRI technologist, etc.).
- Allow an appropriately screened relative or friend to remain with the participant during the MR examination.
- Maintain verbal, visual, and/or physical contact with the participant during the MR procedure.
- Use an appropriate stereo system to provide music to the participant.
- Use an appropriate video monitor or goggles to provide a visual distraction to the participant.
- Use a virtual reality environment system to provide audio and visual distraction.
- Use mirrors or prism glasses to redirect the participant's line of sight.
- Use a blindfold so that the participant is not aware of the surroundings.
- Use bright lights inside of the MR system.
- Use a fan inside of the MR system.
- Use vanilla scented oil or other aroma therapy.
- Use relaxation techniques such as controlled breathing or mental imagery.
- Use systematic desensitization.
- Use medical hypnosis.
- Use a sedative or other similar medication.
-
Acoustic noise protection
-
Bioeffects
Reversible abnormalities include, but are not limited to:- Localized tissue and core body temperature heating
- Tingling sensations
- Peripheral nerve stimulation (involuntary muscle contractions)
- Burns - Burn hazards are caused by damaged hardware or by electrical currents produced in conductive loops of material.
-
Appropriate participant preparation
To prevent excessive heating and possible burns in association with MR procedures, MR Personnel should go through the following checklist in preparing the participant:- Participants should wear attire that does not contain metallic material.
- There should be no unnecessary metallic objects contacting the participant's skin, e.g. drug delivery patches with metallic components, necklaces, bracelets, key chains, etc.
- Prepare the participant for the MR procedure by using insulation material, i.e. appropriate padding, to prevent skin-to-skin contact points and the formation of "closed-loops" from touching body parts.
- Insulation material, i.e. appropriate padding, should be placed between the participant's skin and the transmit RF coil that is used for the MR procedure. Alternatively, the transmit RF coil itself should be padded. There should be no direct contact between the patient’s skin and the transmit RF body coil of the MR system. This is especially important for MR examinations that use the transmit RF body coil or other large RF coils for transmission of RF energy.
-
Appropriate use of equipment
To prevent excessive heating and possible burns in association with MR procedures, MR Personnel should go through the following checklist with regards to the equipment in Zone IV:- Use only electrically conductive devices, equipment, accessories (e.g., ECG leads, electrodes, etc.), and materials that have been thoroughly tested and determined to be safe or otherwise acceptable for MR procedures.
- Carefully follow specific MR safety or MR conditional criteria and recommendations for implants and devices made from electrically-conductive materials (e.g., bone fusion stimulators, neurostimulation systems, cardiac pacemakers, cochlear implants, intracranial pressure monitoring catheters, etc.).
- Before using electrical equipment, check the integrity of the insulation and/or housing of all components including surface RF coils, monitoring leads, cables, and wires. Preventive maintenance should be practiced routinely for such equipment.
- Remove all non-essential electrically conductive materials from the MR system prior to the MR procedure (i.e. unused surface RF coils, ECG leads, EEG leads, cables, wires, etc.).
- Keep electrically conductive materials that must remain in the MR system from directly contacting the patient by placing thermal and/or electrical insulation between the conductive material and the patient.
- Keep electrically conductive materials that must remain within the transmit body RF coil or other transmit RF coil of the MR system from forming conductive loops. Note: The patient's tissue is conductive and, therefore, may be involved in the formation of a conductive loop, which can be circular, U-shaped, or S-shaped.
- Position electrically conductive materials to prevent "cross points". A cross point is the point where a cable crosses another cable, where a cable loops across itself, or where a cable touches either the patient or sides of the transmit RF coil more than once. Even the close proximity of conductive materials with each other should be avoided because cables and RF coils can capacitively-couple (without any contact or crossover) when placed close together.
- Position electrically conductive materials (e.g., cables, wires, etc.) to exit down the center of the MR system, not along the side of the MR system or close to the transmit RF body coil or other transmit RF coil.
- Do not position electrically conductive materials across an external metallic prosthesis (e.g., external fixation device, cervical fixation device, etc.) or similar device that is in direct contact with the patient.
- Allow only properly trained individuals to operate devices (e.g., monitoring equipment) in the MR environment.
- Follow all manufacturer instructions for the proper operation and maintenance of physiologic monitoring or other similar electronic equipment intended for use during MR procedures.
- Electrical devices that do not appear to be operating properly during the MR procedure should be removed from the patient immediately.
-
Closely monitor the participant during the MR procedure
If the participant reports sensations of heating or other unusual sensation, discontinue the MR procedure immediately and perform a thorough assessment of the situation. -
RF surface coil decoupling failures
can cause localized RF power deposition levels to reach excessive levels. The MR system operator will recognize such a failure as a set of concentric semicircles in the tissue on the associated MR image or as an unusual amount of image non-uniformity related to the position of the transmit RF coil.
The operating modes for MR systems as defined by the International Electrotechnical Commission (IEC) are as follows:
Normal Operating Mode
Mode of operation of the MR equipment in which none of the outputs have a value that may cause physiological stress to patients. (Note: The
default specific absorption rate (SAR) level is 2.0-W/kg.).
First Level Controlled Mode
Mode of operation of the MR equipment in which one or more outputs reach a value that may cause physiological
stress to patients, which needs to be controlled by medical supervision.(Note: The default specific absorption rate (SAR) level is 4.0-W/kg.)
Second Level Controlled Operating Mode
Mode of operation of the MR equipment in which one or more outputs reach a value that may produce significant risk for patients, for which explicit ethical approval is required.
-
Pregnancies
- Participants Currently, there are no known biological effects of MRI on fetuses. However, there are a number of mechanisms that could potentially cause adverse effects as a result of the interaction of electromagnetic fields with developing fetuses. Cells undergoing division, which occurs during the first trimester of pregnancy are more susceptible to these effects. The CIA Policy is that all female participants will be required to complete the pregnancy-related questions on the MRI screening form. If the participant is uncertain of her pregnancy status, the imaging study will be delayed until she has received her next menstrual period or decides she is not pregnant. If the participant is determined to be pregnant, the MRI will not be performed, unless the study itself is specifically designed to investigate pregnancy with IRB approval.
-
MR personnel
The CIA recommends that MR Personnel who are pregnant read the following MRI Pregnancy Safety Information to make an informed decision for herself.
-
Children
Children are defined as participants under the age of 13. Children may only enter Zones III or IV as participants in an IRB approved research study of children. Children not involved in the research study (e.g, the child or sibling or a research subject) may not enter Zones III or IV, and must remain under the direct supervision of a parent/guardian to monitor their activities, ensure their safety, and ensure that others working at the Center are not disturbed. Equipment room doors must be kept closed whenever children are present.
All safety precautions applicable to adult subjects are applicable (and if anything, more important) when scanning children. Careful metal screening, accurate entry of age, sex and weight, and use of Normal Operating Mode scanning options whenever possible are important steps in minimizing risks to this population.
One parent or caretaker of a child participant is allowed to enter Zone IV with the child if desired. This parent or caretaker must complete all of the same MR Safety Screening procedures as the child. All studies are encouraged to provide the option of an adult monitor (faculty/staff/student), who is at least MR Operator Level 1, to accompany the child into the scanner room. The accompanying personnel, parent or study-provided adult monitor, should be given acoustic protection and instructed on how to use the safety squeeze bulb.
All researchers who are involved with pediatric scanning are required to attend an additional training session focused on the issues particular to the scanning of children. This will include issues of assent and consent, techniques for increasing compliance in children, testing limits for children, use of a mock scanner, how to deal with anxious subjects, how to determine if an older child needs someone in the room, etc. Attendance at additional refresher training sessions may also be required as the safety policies evolve. -
Patients
Investigators scanning clinical patients take responsibility for precautions proper to relevant specific needs or conditions. -
Participants with poor temperature regulation
When scanning participants with medical conditions such as fever, diabetes, pregnancy, or cardiovascular disease that can impair thermal regulation, the scanner should be in Normal Operating Mode if possible, since energy deposition is not a concern in this mode. Children or elderly subjects are also at increased risk of overheating. If scanning subjects with conditions associated with impaired thermal regulation in "First Level Controlled Operating Mode, investigators and personnel should be attentive to signs or symptoms of overheating and stop the scan if overheating is suspected. First Level Controlled Operating Mode should be avoided if possible in subjects who are unable to communicate reliably (e.g., children, sedated subjects, stroke patients). Adjusting the fan in the scanner may be helpful in reducing the likelihood of overheating in subjects. -
Prisoners
MR scanning of prisoners or parollees with metallic prisoner-restraining devices or RF ID or tracking bracelets could lead to theoretical adverse events, including: (i) ferromagnetic attractive effects and resultant participant injury, (ii) possible ferromagnetic attractive effects and potential damage to the device or its battery pack, (iii) RF interference with the MRI study and secondary image artifact, (iv) RF interference with the functionality of the device, (v) RF power deposition and heating of the bracelet or tagging device or its circuitry and secondary participant injury (if the bracelet would be in the anatomic volume of the RF transmitter coil being used for imaging). Therefore, when scanning a participant, prisoner, or parolee wearing RF bracelets or metallic handcuffs or ankle cuffs, request that the participant be accompanied by the appropriate authorities who can and will remove the restraining device before the MR study and be charged with its replacement following the examination. -
Obese or large subjects
Participants weighing more than 400 pounds should not be scanned. This is the weight limit for the MR compatible gurney that might be needed to transfer the participant off the table during an emergency. The Prisma 3.0 Tesla scanner bed is designed to support weights up to 550 pounds. Even subjects weighing substantially less than 400 pounds should never be allowed to sit at the distal end of either of the scanner beds, since they are not designed to support the full weight of a large subject applied at full mechanical advantage. To avoid burns, a minimum distance of 5 mm should be maintained between the subject's body and the wall of the scanner tunnel. MR pads or cotton sheets available in the MR scan rooms can be used to assure this distance is maintained.
 This indicates equipment is safe for use with MR
This indicates equipment is safe for use with MR This indicates equipment is approved for conditional use with MR.
This indicates equipment is approved for conditional use with MR.
For more information about equipment MR-compatibility certification terminology, see MRISafety.com Terminology Explanation.
-
Acoustic noise protection
Anyone in the scanner room while the scanner is in operation must be provided with and must use hearing protection in the form of earplugs and/or headphones to avoid hearing injury from the acoustic noise generated by the scanner. According to the Prisma documentation, if you are using the Siemens headphones you MUST also provide the subject with earplugs for additional hearing protection. The FDA requires 30 dB or greater for hearing protection and the Siemens headphones alone only provide 13 dB. -
Emergency squeeze bulb
The scanner is equipped with a squeeze bulb that allows the subject to set off an audible alarm to attract the operator's attention. The squeeze bulb should be made available to subjects unless some alternative method of constant monitoring (e.g., another person in the scanner room) is in effect. Use of the squeeze bulb or some comparable form of continuous subject monitoring is mandatory if you are operating the scanner in "First Level Controlled Operating Mode" mode, which has an increased risk of magnetostimulation or subject heating due to RF energy deposition or if you are scanning a subject who has a tattoo or permanent eyeliner. The squeeze bulb plugs into the red connector at the foot of the bed. If the subject squeezes the squeeze bulb, a continuous audible alarm is emitted via the intercom and the intercom alarm button lights up.
 This indicates equipment is NOT approved for use around MR and its presence is a hazard.
This indicates equipment is NOT approved for use around MR and its presence is a hazard.
-
Fire extinguishers
Only the blue and white fire extinguishers marked with the square green "MR Safe" or "MR Conditional" label are safe to bring into the MRI Suite. Never bring a standard red fire extinguisher from elsewhere into the building into the scanner room! -
Automatic External Defibrillator (AED
An automated external defibrillator (AED) is located in the control room. The AED and associated equipment is MR Unsafe and should never be brought into an MR scanner room. A subject in need of resuscitation must be removed from the scan room using the MR Safe or MR Conditional gurney before AED equipment and supplies can be safely used. -
Oxygen Tanks
Both scan rooms are equipped with compressed air and suction from tubes that hang from the ceiling. Medical oxygen is not available in the scan room. Only oxygen tanks made from aluminum are MR Conditional. All others are MR Unsafe.
Individual investigators and their respective IRB policies will determine if incidental findings readings are required.
Quality control, including scanning of a Phantom for scanner calibration and maintenance of equipment, will be performed by an MRI technologist or a member of the MRI Physics group.
All surfaces that have come into contact with a research participant, animal model or any other potentially infective substance must be properly cleaned before the next MRI study is conducted. It is the responsibility of all researchers to prevent any infectious material of research participant or animal model origin from being transferred in any way to another research participant, animal or individual.
-
Cleaning procedures
Follow the written policy that includes cleaning procedures for the MRI environment, as well as a cleaning schedule, that will be posted throughout the center. -
Hand washing
There is a mandatory hand-washing / hand-sanitizing procedure between each participant not only for MRI healthcare workers, but also for others who come into contact with participants. -
Clean the equipment
Clean the MR scanner table, inside the bore of the MR system and other items that come into contact with a participant. Clean all positioning pads and sponges with an approved disinfectant. Infection control experts recommend cleaning after each participant. For participants with any known infectious process, add 10-15 minutes when scheduling scan time to ensure adequate time to thoroughly clean the room and participant-related surfaces. -
Maintenance of padding
Periodically inspect the pads with a magnifying glass, particularly at the seams, to identify fraying or tearing. Replace the pads, as needed. -
Pillows
Use pillows with a waterproof covering that is designed to be surface wiped. Replace pillows when this barrier is compromised. -
Body fluids
Promptly remove body fluids and then disinfect all contaminated areas. -
Open wounds
If the participant has an open wound or history of infection, especially related to MRSA, gloves and gowns must be worn by all staff coming into contact with the participant. These barriers must be removed before touching other areas not coming into contact with the participant (e.g., door knobs, scanner console, computer keyboards, etc.). -
Furniture
All furniture should be periodically cleaned. Ideal surfaces are those that are waterproof, nonporous, and easy to clean. Infection control experts recommend that such cleaning be performed between participants.
Site-specific safety training will familiarize all investigators with the procedures for both controlled and uncontrolled quench of the scanners, and signs in the scanner room and control room explain the procedure. See section below, Uncontrolled Quench.
Both emergency and routine electrical shutdowns will be demonstrated and explained during on-site safety training, and signs in the control room and scanner room explain the procedures.

-
A. Subject medical emergency
In case of medical emergency, call 911. Emergency medical supplies, including AEDs and steel oxygen tanks are MR Unsafe. Use the MR Safe or MR Conditional gurney to move the patient to Zone II before using these supplies. Be aware that medical personnel may not enter the scanner room. -
Subject non-medical emergency
- Claustrophobia or anxiety If a research participant experiences claustrophobia or anxiety during the scan, the scan should be stopped immediately and the MR system Operator should communicate with the participant. If the participant cannot continue, the scan is ended.
-
Tingling or muscle twitches
Tingling or muscle twitches are potential physiologic effects of time-varying magnetic fields. Such effects are particularly likely to occur with echo-planar imaging in fMRI studies. To minimize the likelihood of such magnetostimulation, operate the scanner in Normal Operating Mode. In this mode, only 1% of subjects should experience such effects. However, the scanner may refuse to scan certain subjects with certain pulse sequences in Normal Operating Mode. If you operate in First Level Controlled Operating Mode, up to 50% of subjects may experience magnetostimulation with certain pulse sequences. Complaints of tingling or muscle twitches should prompt rescreening for any metal objects that might have been previously overlooked and verification that subject positioning does not form potential loops. For echo planar imaging, selecting a phase encoding direction that is anterior-posterior (when this is an option) should reduce the likelihood of magnetostimulation. Note that the sensory input associated with magnetostimulation will pose an unwanted confound in fMRI studies.
-
Audible Alarms
You should never scan while an audible alarm is sounding. If you cannot identify and correct the underlying problem, your study should be discontinued.-
Response to squeeze ball alarm
The alarm might have been triggered by someone squeezing the squeeze bulb. Look to see if the participant alarm button on the intercom is lit. If it is, do the following:- If a scan is ongoing, click the "Stop Icon" button on the console using the mouse.
- To stop the audible alarm, press the participant alert button.
- While holding down the intercom talk button, speak to the subject to determine why the squeeze bulb was pressed. Make sure the volume is turned up so that you can hear the subject's response.
- If necessary, enter the room to further investigate and/or correct the problem.
-
Building fire alarm
In case of a fire in the building, please follow the steps below. If you feel that it is necessary to use a fire extinguisher in the scanner room, use the blue and white one designated with the green "MR Safe" or "MR Conditional" sticker. Never use a standard red fire extinguisher from elsewhere in the building.-
Call 9-1-1
Give the location as:
Center For Image Acquisition, 2025 Zonal Avenue, Los Angeles CA 90033 - If smoke or fire is coming from the scanner, equipment room or console, perform the electrical shutdown procedures as demonstrated during your site-specific training and as illustrated by the signage in the scanner control room.
- If you are scanning and smoke or fire is not coming from the scanner, equipment room or console, stop the scan by clicking the stop icon in the bottom left of the console screen with the mouse.
- Remove the subject from the scanner using the home button on the gantry and escort the subject out of the building.
- If time permits, initiate the electrical shutdown procedures.
- Do not return to the building until advised by personnel that it is safe to do so.
- Contact the CIA Director or a member of the MRI Safety Committee to advise them that there was a fire in the building.
-
Call 9-1-1
-
Helium level too low in the room
Check the Siemens control box located in the control room to see whether the helium level is too low. If the warning LED is lit, there could be a potential issue such as low helium, power problems, compressor problems, battery problems and/or communications errors. You can press the alarm silence, but do not scan. Notify CIA Director or a member of the MRI Safety Committee and send your participant home. -
Uncontrolled quench
Site-specific training covers both controlled and uncontrolled quench, and signs in the scanner room outline procedure. -
Low oxygen level alarm
The oxygen monitors will emit an audible alarm if the oxygen level drops to 19.5%. An LED will light up on the front panel, and the display will alert you to the oxygen level AND/OR the error message. If the alarm sounds and the display indicates a low oxygen level, immediately check on your subject, open the door to the scanner room and remove the subject from the scanner room in the safest manner possible (i.e. MR safe gurney or MR safe wheelchair if needed). If your subject needs medical attention, call 911. Please alert the CIA Director or a member of the MRI Safety Committee immediately if there is a decrease in oxygen in the scanner room. -
Other errors
It is safe to scan if the oxygen levels are normal. If a voltage or surge problem occurs, it is possible that the device will alarm, that the LED light will come on AND/OR that an error message will be displayed, despite normal oxygen levels in the room. In this case, verify that your subject is fine and ALWAYS verify that the oxygen level is normal (20.8-20.9%) before resuming scanning. If the error is non-oxygen related, please contact the CIA Director or a member of the MRI Safety Committee by email to let them know the details of the problem.
-
Response to squeeze ball alarm
-
Door Failures
Switches on both sides of the scanner room doors operate the pneumatic devices that assure that the doors are appropriately sealed against radiofrequency leaks. Both switches must be in the up position for the door to seal. You should not scan if the doors do not properly seal since your data can be potentially contaminated. It is possible in the event of a door malfunction that you might be unable to open the door and if you are inside the scanner room you might find yourself trapped. You should never close the scanner door from the inside if no one is on the outside to provide assistance should this occur. If no other means of timely escape is available, it may be necessary to forcefully disconnect the tubing that operates the pneumatic seal or to break the glass between the scanner room and corresponding control room. -
Electrical Fire
In the event of smoke or flames are detected in the vicinity of the electrical equipment, the operator should press the red Emergency Power Shutdown button (NOT the red quench button) located in the control room or in the magnet room. Follow standard evacuation procedure (see below).
-
Uncontrolled Quench
In the event of a spontaneous quench of the MRI system there is a total venting of the liquid helium. Be aware that cryogenic gases may vent partially or completely into the scan room, as evidenced in part by the sudden appearance of white clouds or fog around or above the MR scanner.
Follow these steps:- Remove research participant and others from MRI system room.
- Secure magnet room door. The MRI system operator is responsible to ensure no one enters scanner room without proper screening for MRI safety. Note that even in the event of a quench, a significant magnetic field may remain for some period of time.
- After ensuring the magnet and equipment rooms are secure and that all individuals have exited these areas, inform Siemens of the quench and contact the CIA Executive Committee. A written report of the circumstances will be required.
-
Evacuation Procedure
In the event that the MRI scanner and control room must be evacuated, follow these procedures:- Safely remove the participant from the scanner
- The MRI-operator is required to securely lock the door to the control room to ensure that no emergency personnel or unscreened emergency equipment are accidentally exposed to the standing magnetic field of the MRI system
- All personnel should evacuate the building through the nearest exit
- Do not reenter the building until granted permission by the Fire Department.
-
MRI System Malfunction
In the event that the MRI system malfunctions, the MRI-operator must log a service call to the service arm of Siemens Medical Solutions by calling Siemens at 800-888-7436. Non-exhaustive reasons for logging a service call include the following, failure to boot or reboot the MRI system; system default messages; magnet stop alarms; and chiller malfunctions.

For all medical and safety emergencies dial 9-1-1.
If there are any doubts regarding whether an individual may be safely scanned, do not allow the individual to enter the scanner room.
It is strongly recommended that you screen participants for MR contraindications well in advance of the scanning date. Some implants and devices have been established as MR Safe or MR Conditional for MR scanning. A recent copy of Reference Manual for Magnetic Resonance Safety, Implants and Devices, cataloguing implanted medical devices is available in the MR suite and up-to-date information is always available on the website http://www.mrisafety.com. Additionally, you may find information at the manufacturer's website, or receive written approval stating that the participant is safe to participate in a research MRI from the participant's physician. If, in reviewing these resources, you believe that it is possible to safely scan your subject, you should contact the CIA Executive Committee to request authorization to scan the subject. Please allow adequate time for the committee to review requests. Even if you are certain that the implant or device constitutes a risk,do not allow the individual into the scanner room unless you have obtained explicit authorization to do so.
-
Implants and Medical Devices
These will be evaluated on a case by case basis following the recommendations of the involved physicians and of the guidance of MRISafety.com. -
Post-operative conditions
These will be evaluated on a case by case basis following the recommendations of the involved physicians and of the guidance of MRISafety.com. -
Past eye injuries
Participants with history foreign bodies in the eyes will require a negative CT scan of the orbits prior to being admitted to Zones III or IV. -
Tattoos
Tattoos are not an absolute contraindication for MRI procedures. Heavily tattooed individuals, particularly of the head and neck should be instructed to be alert for any heating sensations and to notify the magnet operator (by using the squeeze ball) should they experience any discomfort. Participants with tattoos may or may not have an MRI based on the opinion of the magnet operator or the researcher.
For participants with extensive or dark tattoos, including tattooed eyeliner, to decrease the potential for RF heating of the tattooed tissue, it is recommended that cold compresses or ice packs be placed on the tattooed areas and kept in place throughout the MRI process if these tattoos are within the volume in which the body coil is being used for RF transmission. This approach is ACR Guidance on MR Safe Practices 513 especially appropriate if fast spin echo (or other high RF duty cycle) MRI sequences are anticipated in the study. If another coil is being used for RF transmission, a decision must be made if high RF transmitted power is to be anticipated by the study protocol design. If so, then the above precautions should be followed. Additionally, participants with tattoos that had been placed within 48 h before the pending MR examination should be advised of the potential for smearing or smudging of the edges of the freshly placed tattoo. -
Skin staples
Skin staples and superficial metallic sutures: Participants requested to undergo MR studies in whom there are skin staples or superficial metallic sutures (SMS) may be permitted to undergo the MR examination if the skin staples or SMS are not ferromagnetic and are not in or near the anatomic volume of RF power deposition for the study to be performed. If the nonferromagnetic skin staples or SMS are within the volume to be RF irradiated for the requested MR study, several precautions are recommended:
a. Warn the participant and make sure that they are especially aware of the possibility that they may experience warmth or even burning along the skin staple or SMS distribution. The participant should be instructed to report immediately if they experience warmth or burning sensations during the study (and not, for example, wait until the end of the knocking noise).
b. It is recommended that a cold compress or ice pack be placed along the skin staples or SMS if this can be safely clinically accomplished during the MRI examination. This will help to serve as a heat sink for any focal power deposition that may occur, thus decreasing the likelihood of a clinically significant thermal injury or burn to adjacent tissue. -
Drug delivery patches
Some drug delivery patches contain metallic foil. Scanning the region of the metallic foil may result in thermal injury. Because removal or repositioning can result in altering of patient dose, consultation with the patient's prescribing physician would be indicated in assessing how to best manage the patient. If the metallic foil of the patch delivery system is positioned on the patient so that it is in the volume of excitation of the transmitting RF coil, the case should be specifically reviewed with the radiologist or physician covering the case. Alternative options may include placing an ice pack directly on the patch. This solution may still substantially alter the rate of delivery or absorption of the medication to the patient (and be less comfortable to the patient, as well). This ramification should therefore not be treated lightly, and a decision to proceed in this manner should be made by a knowledgeable radiologist attending the patient and with the concurrence of the referring physician as well. If the patch is removed, a specific staff member should be given responsibility for ensuring that it is replaced or repositioned at the conclusion of the MR examination. -
Aneurysm clips
Participants with a cranial aneurysm clip will require a written report from the referring physician stating the name of the clip and the date of placement prior to being admitted to Zones III or IV. Only patients with MR Safe or MR Conditional aneurysm clips are allowed to undergo an MRI procedure.