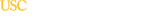The information below summarizes important operating procedures for the CIA core facility. It is intended as a resource for investigators, but is not an exhaustive list of all CIA capabilities and policies. If you find that your questions are not answered here, please contact us at cia@ini.usc.edu.
Click an image for details. Click again to close.
Investigators submitting a grant are strongly encouraged to contact cia@ini.usc.edu with the details of their research plan prior to grant submission, ideally with at least 2 weeks lead time. This is especially true for PIs who have never scanned at CIA before, as our resources and policies may differ from those of Keck Medical Center and other institutions. If you are new to MR imaging, please be aware that each scanner and coil system is unique, and replicating scan parameters developed or published elsewhere may or may not be possible. We are happy to help review your proposal to ensure that it will be technically and logistically feasible at CIA. We can also provide estimated costs and a letter of support for your proposal if desired.
Please see the overview of our current facilities and equipments that may be useful in preparation of Facilities and Other Resources sections.
The Center for Image Acquisition (CIA) has a 3 Tesla, Siemens Magnetom Prisma MRI system and a 7 Tesla, Siemens Magnetom Terra MRI system. These scanners are among the most advanced imaging devices approved for use on human subjects, and with them, the CIA and related researchers push the boundaries of possibility in terms of spatial and temporal scans of the brain. Both foundational research and clinical scans are performed at the CIA and the facility is equipped with knowledgeable staff as well as the latest in advanced equipment.
CIA first floor | Server lobby
CIA first floor | MRI scanner room

3T Siemens Magnetom Prisma MRI System
The Prisma 3T is a high performance MRI system based on the Trio system. It allows for unparalleled simultaneous 80mT/m @ 200T/m/s gradients. Its 60 cm bore diameter allows for magnet homogeneity at 40 cm DSV – 0.2 ppm. With 64 – 128 channel receiving, it is the ultimate MRI system for neuroimaging. The Prisma offers standard Diffusion Spectrum Imaging up to 514 diffusion directions, allowing characterization of crossing fibers. It is also capable of higher resolution DTI with a reduced field of view – ZOOMit DTI for isotopic DTI. Higher SNR also allows for reproducible 3-Dimensional, Arterial Spine labeling (ASL) perfusion and improved resting state fMRI data.
The Magnetom Prisma MRI system is FDA-approved, which allows its high-resolution capabilities to be used in the clinical imaging of patients with neurological diseases and other conditions affecting the brain. The key aspect of this system that is unique is its powerful XR Gradients, which have a strength of 80 milliTesla per meter (mT/m) combined with a 200T-per-meter-per-second (T/m/s) slew rate. Furthermore, it employs advanced homogeneity and shimming functions for optimal MRI data acquisition.
3T Technical Specifications
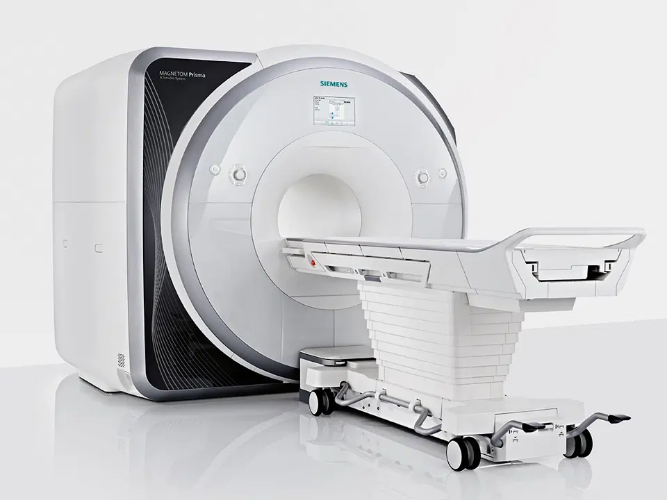
7T Siemens Terra MRI System
The Siemens MAGNETOM Terra is the first 7T MRI scanner for diagnostic imaging and is designed for unprecedented breakthroughs in clinical care. This advanced ultra-high-field (UHF) technology is an actively shielded whole body highly homogeneous superconducting magnet, which was installed at the CIA in March 2017. This unique scanner is designed for both research and clinical applications and can be switched to clinical diagnostic imaging tasks with a Dual Mode feature in less than 7 minutes. A comparison of the two modes is available in the table below.
| RESEARCH MODE | CLINICAL MODE |
|---|---|
| syngo MR E11 software line | syngo MR E11 software line |
| XR gradients 80/200 | XR gradients 80/200 |
| Up to 64 receiver channels | Up to 64 receiver channels |
| MaRS computer | MaRS computer |
| 8 channel parallel transmit | 1 transmit channel |
| 8 x 2 kW RF power | 11 kW RF power |
| Wider range of RF coils | 2 coils available (head and knee) |
The Terra 7T utilizes an actively-shielded, highly homogeneous superconducting magnet housed in an 830 mm horizontal room temperature bore, low-loss helium cryostat, and zero helium boil-off technology. Field shimming is primarily accomplished using superconducting shim coils. Final shimming is performed with a small number of passive shims. This scanner is supplied with a helium level monitor and an emergency quench heater control unit. A two-stage 4.2 K cryo refrigerator cooling system is employed, consisting of two independent cryocooler systems that eliminate the static cryogenic consumption.
The gradient system essentially consists of a Gradient Power Amplifier (GPA) and a Gradient Coil (GC), both water-cooled to sustain a high duty cycle. The Magnetom 7T uses the GPA model similar to that used in other Magnetom MRI systems. The XR gradients are the same as the Prisma, capable of 80mT/m @ 200T/m/s gradients. The gradient coil includes the full set of five second-order electrical shim coils to adjust static magnetic field homogeneity for subject or patient and each measurement volume. The Siemens RF receiver technology uses 64 channels, and the 7T Terra at USC includes a Nova Medical 32-channel head coil. This MRI system also has an 8-channel parallel RF transmit array (pTx). Third order shims provide four out of the seven possible third order shims built into the SC72 gradients, along with second 4-fold SPS cabinet, wiring for third order shim, electric infrastructure expansion, and an SW interface to drive additional third order shims. d can be switched to clinical diagnostic imaging tasks with a Dual Mode feature in less than 7 minutes. A comparison of the two modes is available in the table below.
The 8Tx-32Rx head coil is the very latest of Nova Medical's high-performance products for the Siemens Whole-body 7T MRI scanner. It features a new highly efficient 8Tx transmit shell. By enabling B1 shimming or full parallel transmission (pTx) operation, the new 8Tx array allows greatly improved B1 transmit characteristics, which increase both contrast and sensitivity in regions of the brain not well served by a single-channel transmit coil. By combining this state-of-the-art transmit shell with the well-known thirty-two channel receive array, optimal image quality is achieved over the full brain including the cerebellum, hippocampus, and deep brain structures.
Available 2023. A flexible body coil, originally developed by University of Queensland and commercialized by UniQuest. This array coil has a flexible modular design consisting of 8 individual antenna elements that allows it to be conformed across multiple body regions. It also offers a high efficiency for producing transmit B1 fields within the SAR limit, and is compatible with the 8-channel parallel RF transmission (pTx) system of the 7T Terra for overcoming B1 field inhomogeneities.
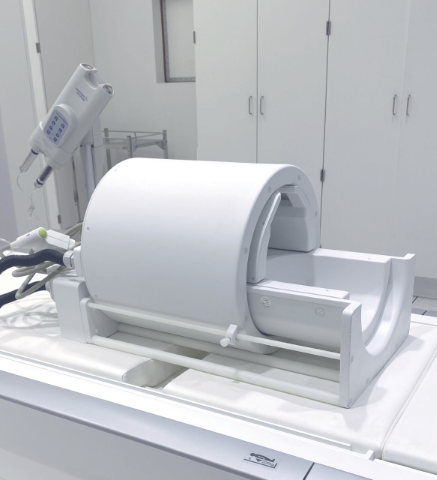
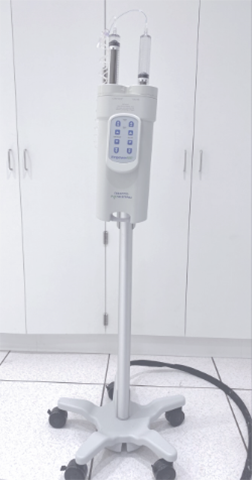
| MRI Coils Nova Medical Head | Knee Coil | 7T Power Injector |
|

|
|
The Center for Image Acquisition has a variety of equipment to help you conduct an MRI procedure and to ensure the comfort of your study participants and/or patients. This equipment includes the latest in scanning and data acquisition technology, to MR Conditional physiological monitoring equipment as well as MR Safe or MR Conditional emergency medical supplies. If you have questions about equipment that aren't answered here, please email cia@ini.usc.edu.
Private consultation rooms
Blankets & linens
Gowns & scrubs
Ear plugs
Blanket warming cabinet
Medical Equipment
Philips Healthcare cardiac defibrillator with aspirator pump
MR Conditional oxygen tanks
MR Conditional Transport Stretcher
MR Conditional Wheelchair
MR Conditional walker
MR Conditional Exam Stool
MR Conditional IV Stand
MR Conditional Exam Light
Pyxis MedStation
Sensimetrics Model S14 headphones (3T)
Sensimetrics Model S15 headphones (7T)
Medrad Spectris Solaris Power Injector (3T)

Physiological Monitoring
BioPac Systems MR Physiologic monitor - MP160 System - Ethernet-ready hardware and software solution for data acquisition and analysis, capable of recording up to 16 channels at once with different sampling rates and recording at speeds up to 400 kHz (aggregate).
Please visit the manufacturer site for more information
Philips Invivo Expression MR400 Patient Monitor - Bedside-quality patient monitoring of anesthetic agents, body temperature, advanced cardiac architecture and superior ECG signal and wireless gating, position flexibility, F01 Basic setup (noninvasive blood pressure, ECG, Oxygen Saturation, Respiratory Rate), alarm flags, 15" LED widescreen viewing.
View technical specifications here
Console and Computer Equipment
The control rooms associated with the MRI systems are equipped with iMacs capable of running Windows.
A Fuji PACS workstation is available to any physician with access to Keck’s PACS.
Stimulus Presentation
Visual presentation through Cambridge Research BOLDscreen 32 - 32-inch 1920x1080 widescreen LCD display, up to 16-bit RGB color control, 1400:1 contrast ratio and 120Hz panel drive with integrated calibration.
View technical specifications here
Siemens headphones for audio stimulus presentation are available but are only compatible with the 20-channel, not 32-channel, transmit/receive RF coil. Investigators may bring their own stimulus equipment, as needed, as long as it has been tested and labeled MR Conditional at 3T and/or 7T.
Noninvasive Neuromodulation
The Noninvasive Brain Stimulation (NIBS) Center is directly adjacent to the 3T and 7T MRI systems and provides state-of-the-art equipment for neuronavigation-guided, noninvasive brain stimulation (NIBS) including:
Frameless neuronavigation using a stereotactic image guidance system to facilitate positioning of the TMS coils over a subject's brain using MRI/fMRI (Rogue Research Brainsight™ Frameless system with mobile computer; https://www.rogue-research.com)
Single- and paired-pulse transcranial magnetic stimulation (TMS; Magstim BiStim2 composed of 2 linked 2002 units; http://www.magstim.com/product/20/magstim-bistim2)
Wireless, 8-channel transcranial electrical stimulation (tES) capable of transcranial direct current stimulation, transcranial alternating current stimulation (tACS), and transcranial random noise stimulation (tRNS) and simultaneous electroencephalography (EEG; Neuroelectrics StarStim8; http://www.neuroelectrics.com/products/starstim/starstim-8/)
Wireless, 4-channel surface electromyography (sEMG; Delsys Trigno Lab with 2 Trigno Standard Sensors and 2 Trigno Mini Sensors; http://www.delsys.com/products/wireless-emg/trigno-lab/)
Data acquisition software and hardware to synchronize TMS pulses with sEMG collection (CED micro1401; http://ced.co.uk/products/mic3in; CED Signal v. 6 software; http://ced.co.uk/products/sigovin )
MR Conditional high-definition transcranial electrical stimulation (Soterix; 5-channel HD-tES system: https://soterixmedical.com/research/monitoring/fmri).
Additional capabilities, including repetitive and theta-burst transcranial magnetic stimulation, may be arranged upon request.
INI Stevens Hall | The Center for Image Acquisition (CIA)

The MR environment carries significant risks, and all users should review and understand the safety policies and procedures of the CIA. These are available in a white binder labeled "CIA Policies and Procedures'' in the 3T control room. Any questions about safety procedures should be directed to the MR Technologist or CIA Medical Director. If there is ever any uncertainty about the safety of a device or object within the MR environment, it must not be permitted to pass beyond Zone II. The magnet is always on.
To ensure the safety of all those utilizing the CIA facilities, only Level 2 MR Personnel who are properly trained and certified in MRI safety are permitted to operate the MRI system or to enter sensitive areas of the CIA unsupervised. Certification of MRI safety involves both general online education and testing regarding the risks involved with MRI as well as in-person, site-specific training on our equipment.
All MRI examinations must be conducted by a minimum of two individuals with certification in MRI safety, with at least one individual certified in MRI safety as Level 2 MR Personnel. The CIA MRI Technologist may fulfill the role of the Level 2 MR Personnel during normal scanning hours, as explained below. This means that during regular business hours, at least one additional person must be present who has been certified as at least Level 1 MR Personnel.
Level 1 and level 2 safety certification (described further below) may be initiated by logging into your CIA account. Certificates from other facilities are not accepted, and those from CIA are themselves non-transferrable. Certification establishes only that the holder has completed training related to safety within the MR environment; it does not imply that the holder can operate the scanner effectively, troubleshoot problems, or obtain quality images.
Level 1 MR Personnel
Level 1 MR Personnel are defined by the American College of Radiology as: “Those who have passed minimal safety educational efforts to ensure their own safety and the safety of other staff members as they work within Zone III will be referred to henceforth as MR Operator - Level 1 MR Personnel.” The American College of Radiology defines Zone III as the region in which free access by unscreened non-MR personnel or ferromagnetic objects or equipment can result in serious injury or death as a result of interactions between the individuals or equipment and the MR scanner’s particular environment. These interactions include, but are not limited to, those with the MR scanner’s static and time varying magnetic fields. All access to Zone III is to be strictly physically restricted, with access to regions within it (including Zone IV, the MRI system room) controlled by, and entirely under the supervision of, MR personnel in order for an MRI examination to be performed.
Level 2 MR Personnel
Level 2 MR Personnel are defined by the American College of Radiology as: “Those who have been more extensively trained and educated in the broader aspects of MR safety issues, including, for example, issues related to the potential for thermal loading or burns and direct neuromuscular excitation from rapidly changing gradients, will be referred to henceforth as Level 2 MR Personnel. It is the responsibility of the MR medical director not only to identify the necessary training, but also to identify those individuals who qualify as Level 2 MR Personnel. It is understood that the medical director will have the necessary education and experience in MR safety to qualify as Level 2 MR Personnel.”
In addition to fulfilling the requirements for Level 1 MR Personnel, Level 2 certificate applicants must fulfill the experience requirement by conducting at least 10 full MRI examination sessions (minimum 1 hour each) under the supervision of a Level 2 MR Personnel user or the MRI technologist, with each session (date, time, scan number, scanning protocols used) signed off on by the supervising party. Level 2 MR Personnel training requirements must be met for each scanner used (note, there is separate training required for 3T and 7T MRI systems).
All Level 2 MR Personnel user applications may be subject to an oral examination and/or hands-on competency exam at the discretion of the MR Medical Director and/or the Head of the MRI Safety Committee. All applications for Level 2 MR Personnel user status will be reviewed and approved by the MR Medical Director and/or the Head of the MRI Safety Committee. Level 2 MR Personnel user status must be renewed every two years.
- The PI or Project Administrator must first create a CIA account.
-
Once logged into your CIA account, choose the option to "Request a new project" and provide the required information. Four sets of requirements must be met and will be verified by CIA staff before you are able to scan:
- Regulatory: IRB/IACUC approval
- Financial: WorkTags Cost Center and PPGG or CIA Pilot approval
- Technical: Scan parameters tested by our MR technologist (kmartin@ini.usc.edu)
- Personnel: At least one person in your group must be designated a Monitor and have [MR Level I safety certification].
-
Once all requirements are satisfied, the personnel designated as Schedulers for the project will have access to the CIA scheduling application to book scan slots. The following policies apply:
- Etiquette: All investigators are encouraged to schedule scans only as needed (do not book slots to hold them "just in case"). It is recommended that a single project does not reserve more than 10 slots at any given time. Note the cancellation policy described below. Special requests for advanced scheduling / set scan times may be requested and will be reviewed by CIA administration. Possible requests involve anticipated future scans for longitudinal, clinical trial studies with a set time interval for pre/post scans. The policy for scheduling etiquette is subject to change based on use and demand.
-
Pricing: Prices include scanner time and technologist time during normal business hours only. Scanner time includes time required to set up the scan, get the participant in/out of the scanner, administer contrast, or any other study-related activities requiring the scanner time or technologist time. There are no discounts for scans that do not require a technologist.
- Rates: Scan prices may differ depending on the funding source (commercial vs federally-funded). Test or protocol development scans are billed at the same rate as a regular scan and must be scheduled with your project. Projects include 1 hour of protocol development to be conducted during off-peak hours. All other services are billed separately at our cost.
- Cancellation Policy: Cancellations at least 24 hours in advance will not be charged. Cancellations within 24 hours of the scheduled scan will be charged a fee of 25% of the scan cost. This includes, but is not limited to, cancellations due to participant no-show, scheduling error, illness, and traffic/transportation issues. CIA staff may cancel or modify scanning reservations at any time for any reason, but will usually only do so in coordination with study personnel. Cancellations required by CIA or scanner malfunction will not be charged. Excessive cancellations of more than 5 cancellations within any 30-day period may result in limited scheduling access for the user and/or penalty fees. Questions or concerns about billing for scans or cancellations should be directed to cia@ini.usc.edu.
The CIA Executive Committee recognizes the need for pilot data to support grant applications and will award up to 10 hours of scan time per PI per year for this purpose.
Who is eligible?
- All tenured, tenure track, and Research, Teaching, Practice, and Clinical (RTPC) faculty, and research scientists may act as Principal Investigators. Voluntary faculty may not serve as Principal Investigators.
- Postdoctoral Research Associates and Postdoctoral Teaching Associates, as defined by USC’s Postdoctoral Scholars Policy, may act as co-principal investigators, but may not be principal investigators.
What kinds of studies are eligible?
- Pilot scans are awarded for human subjects only, not animal studies.
- Commercial entities may not use the pilot process directly or indirectly to evaluate their product(s). PI conflicts of interest and consulting relationships must be disclosed.
Other Requirements
- IRB approval is required prior to any scanning.
- Awarded pilot scan hours expire 1 year after the date of your award letter.
- Scan hours canceled or rescheduled with less than 24 hours notice cannot be replaced.
- Additional costs are applicable for services beyond scan time (e.g., MRI contrast agent, review of incidental findings, etc.).
- You must provide a brief progress report on your project no later than three (3) months after the end of your last scan.
- If your pilot data produces a successful grant application, those subsequent funded scans must be performed at the CIA.
- Any publications resulting from these scans must acknowledge the Center for Image Acquisition, USC Stevens Neuroimaging and Informatics Institute.
The Center for Image Acquisition is committed to protecting patient and subject information and securely storing data. The CIA is also committed to fostering collaborative science by encouraging and facilitating use of the Image and Data Archive, a secure data sharing and storage platform powered by the USC Laboratory of Neuro Imaging.
Data Sharing and Security Policy
Protecting the integrity of personal information is of paramount importance to the CIA. The MRI Console computer will not connect to
the internet, nor will any user be allowed under any circumstances to connect any external drive. Data acquired on the MRI console will be
automatically reconstructed and synced to the CIA DICOM Server, and investigators will be able to download their scans from that server via an
email link they’ll receive after each scanning session.
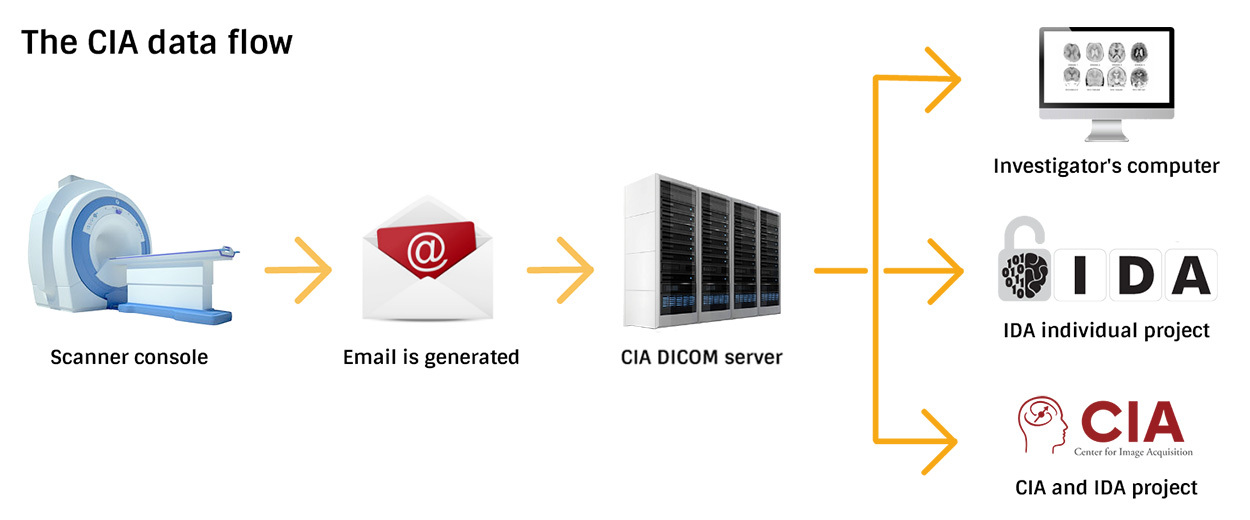
The CIA DICOM Server
Scans from both the 3T and 7T scanners, in DICOM format, undergo de-identification and are given a unique ID as they are passed from the MRI Console to the CIA DICOM server.
The CIA DICOM server exists to protect data and to insulate the scanner consoles from outside connections, and is not intended to house data for long periods of time. This server is behind a firewall, and a download link and CIA login are required to download scans. Scans will be stored on the CIA DICOM server for 2 weeks. Long-term storage in the LONI Image and Data Archive (IDA) is available. The CIA is not responsible for data that has not been sent to an IDA project after 2 weeks.
Data Sharing and the LONI IDA
All subjects and studies are automatically enrolled to share de-identified data through the LONI Image and Data Archive (IDA) database unless PIs elect to opt-out. PIs are able to opt-out of this data sharing mechanism on a per-scan basis.
Investigators also have the option of using the IDA resources to store their data in their own individual "Project" within the IDA, and can elect to send their scans directly from the CIA to their IDA project. IDA projects are access-restricted and investigators have full control over who can view or use their data, and what (if any) sharing they want to enable. Investigators sending data to their own project are able to assign research subject identifiers as part of the send process.
Investigators who elect not to store their data in the IDA receive an email after each scanning session with a link to download their data from the CIA DICOM Server.
More information about the IDA
Setting up your own IDA Project will require you to complete and submit an IDA Study Intake Form and all of the additional information requested on the form at least two weeks in advance of your first scheduled scan. For further information, you may email dba@loni.usc.edu or call (323) 442-7246.
An IDA user account is required for accessing data within the IDA. Setting up an IDA account (to view existing IDA projects) is free and can be done from ida.loni.usc.edu.
Payments to research subjects should be made via one of the mechanisms listed below. Investigators and coordinators should not pay subjects with cash or prepay out-of-pocket. Payments made by investigators to subjects that do not adhere to university policies and practices will not be reimbursed.
Prepaid One-time Card
The Prepaid One-time Card is a debit card issued by Swift Prepaid Solutions, Inc., to facilitate a single payment to individuals who participate in university clinical trials, research studies or incentive programs. More information can be found on the Prepaid One-time Card page. (USC NetID Log-In may be required to view this page.)
Prepaid Recurring ClinCard (ClinCard)
The Prepaid Recurring ClinCard (ClinCard) is a reloadable debit card issued by Greenphire, Inc., to facilitate recurring payments to participants of university clinical trials, research studies or incentive programs. More information can be found on the Prepaid Recurring ClinCard page. (USC NetID Log-In may be required to view this page.) Please note that as of January, 2017, the Keck School of Medicine is still sponsoring ClinCard fees (both card fee and load fee), but this sponsorship is subject to termination at any time and without notice. Please budget accordingly.
Special instructions:
- You must obtain Department Administrator approval for use of either card. Prior to submitting your request via eMarket, please send an email to office@ini.usc.edu to request approval. Once approval is received, make a PDF of the approval email and include the PDF in the “Attachments” tab of the online form. Your card request will not be processed by Corporate Card Services without this approval.
- When submitting the eMarket request for either card, be sure to select “Medicine, Keck School of” as your “Department.”
- Please check with your INI research administrator for your account number and object code. You may do so by sending an email to office@ini.usc.edu.
March 22, 2022
By Sidney Taiko Sheehan
The Keck School of Medicine of USC’s Mark and Mary Stevens Neuroimaging and Informatics Institute (USC INI) is home to the Center for Image Acquisition (CIA), which provides high-resolution high-field imaging capabilities for both neuroscientific research and clinical applications. Opened in 2016, the CIA is an MRI facility housing a Siemens Magnetom Prisma, a 3 Tesla MRI scanner, and a Siemens Magnetom 7T MRI scanner. These scanners include unparalleled innovations for neuroimaging.

Priya Rajagopalan, MBBS, MPH, of the Center for Image Acquisition at the USC Mark and Mary Stevens Neuroimaging and Informatics Institute. (Photo USC/INI)
“The CIA combines two of the world’s most advanced MRI scanners with dedicated supercomputing systems, cutting-edge analysis techniques, and expertise to image, map, and model the structure and function of the living human brain with unprecedented detail,” says USC INI Director Arthur W. Toga, PhD. “It is with immense pleasure that I announce the appointment of Priya Rajagopalan as the CIA’s new medical director.”
For Assistant Professor of Clinical Radiology, Priya Rajagopalan, MBBS, MPH, her new role is like a homecoming. Early in her academic career, she served as a research associate for the Laboratory of Neuro Imaging (LONI), which was launched by Toga in 1983 and became a part of the INI in 2013. “It’s truly meaningful to have researchers join us early on, go elsewhere to hone their skills, and then return in some new capacity. It reinforces our culture here at the INI and shows the strength and diversity of our work,” says Toga.
“From early on during medical school, I knew I wanted to study the brain and I considered neurology,” says Rajagopalan. “It was during my time at LONI that I started working on research projects and became fascinated with neuroimaging. INI Associate Director Paul M. Thompson was my mentor at the time and his encouragement and enthusiasm inspired me to take the path that led me here today.”
As medical director, Rajagopalan will oversee all clinical scans performed at the CIA. She will coordinate with clinical researchers on various imaging projects, while ensuring any scans from research participants that may show the need for acute medical intervention, are effectively managed.
“The CIA houses specialized tools and equipment enabling a variety of imaging techniques to be employed for interdisciplinary research,” notes Rajagopalan. “Though research has been the primary focus, we’re excited to expand further into the clinical realm.”
Rajagopalan also serves as the assistant program director for neuroradiology fellowships at the Keck School. She collaborates closely with residents and fellows across USC and is enthusiastic about mentoring others to help them find their calling. “I see my new role at the CIA as another opportunity to get the next generation interested in neuroimaging. With these fantastic resources at our fingertips, the CIA is becoming a hub for experiential learning, research, and clinical investigation.”
For more information about how you can use the Siemens Magnetom Prisma, the 3 Tesla MRI scanner, or the Siemens Magnetom 7T MRI scanner for your own research, please contact Sidney Taiko Sheehan at ssheehan@ini.usc.edu.